I had the great opportunity with experimenting with various methods of home electrical power productions techniques with the aid of my research and studies. I am happy to share
with you folks with my findings and results.
Let me explain how it all first started.
I came to reason that perhaps I could use the method of Sea "salt" water magnesium cell. I was able to reproduce this kind of technology very simply with a sea water car education
kit I found on eBay. I trashed all the other
parts. I slightly modified the "fuel cell" part and replicated ample
amounts from that template so that I could also experiment with stacking
these cells for more power. My goal was to use the power from these green energy cells to feed an electrolyzer that would indeed produce hydrogen from a tank of water. I would then
feed this hydrogen to a hydrogen cell and
experiment with hydrogen power production at home. How ever, I did
not have any lab equipment as such, until I found a great deal online
from a surplus university sale. I got a whole
kit normally worth thousands! still manufactured today as I looked up
the company. This was almost like new. The kit had all the pipes,
Tubes, hydrogen fuel cells stacks, Various connection wires, meters, lamps motors etc,,, In one long story short. I had everything to experiment with this kind of power right here at
home!
First I had to take a look back to basics!
A simple battery can be made for the purpose of education. Typically, a piece of zinc metal (such as a galvanized nail) and a piece of copper (such as a penny) are inserted into a
lemon and connected by wires. Power generated by reaction of the metals is used to power a small device such as a light emitting diode (LED).
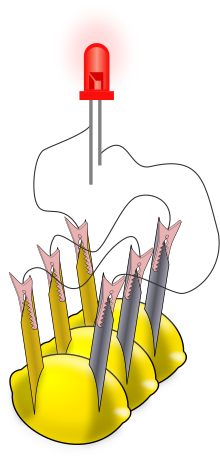 The
lemon battery is similar to the first electrical battery invented in 1800
by Alessandro Volta, who used brine (salt water) instead
The
lemon battery is similar to the first electrical battery invented in 1800
by Alessandro Volta, who used brine (salt water) instead
of lemon juice. The lemon battery illustrates the type of chemical reaction (oxidation-reduction) that occurs in batteries. The zinc and copper are called the electrodes,(Cathode And Anode)
The juice inside the lemon is called the electrolyte. There are many variations of the "fruit" cell that use different fruits (or liquids) as electrolytes and metals other than zinc and copper as
electrodes.
The lemon battery is similar to the first electrical battery invented in 1800 by Alessandro Volta, who used brine (salt water) instead of lemon juice. The lemon battery illustrates the type of
chemical reaction (oxidation-reduction) that occurs in batteries. The zinc and copper are called the electrodes,(Cathode And Anode) and the juice inside the lemon is called the electrolyte.
There are many variations of the "fruit" cell that use different fruits (or liquids) as electrolytes and metals other than zinc and copper as electrodes. I have experimented with various types
and will discuss the outcomes in a moment.
A typical voltage is 0.9 V with lemons. Currents are more variable, but range up to about 1 mA (the larger the electrode surfaces, the bigger the current). For a more visible effect, lemon
cells can be connected in series to power an LED or other low current devices. Note that with the fruit battery setup, Incandescent light bulbs from flashlights are not used because the
lemon battery is not designed to produce enough electric current to light them. How ever. substituting different metals as the positive anode electrode will yield better results. Such as
using magnesium instead makes a cell with a larger voltage (1.5−1.6 V) and much more current output. In some cases, A single cell could light up an incandescent light bulb. Using the
magnesium setup instead. More on that later,
The energy comes from the chemical change within the anode "positive" electrodes. Not the fruit itself. oxidized inside the lemon. This reaction is called oxidation. The anode atoms
dissolve into the liquid electrolyte as electrically charged ions, exchanging some of its electrons with the acid in order to reach a lower energy state,While (dissolving) zinc anode atoms
are entering the cathode electrolyte and the energy released
provides the power.
Note: "tap" water may work somewhat as the "acid" electrolyte carrier, as
your most likely going to have a weak but measurable PH level in the water
from various treatment chemicals etc...
Expect almost the same result as the lemon battery "acid", Simply using tap water in a cup and the 2 electrodes soaked in the cup. You should get a reading of around 0.9 Volts DC. As I have
with variations of this experiment, using nothing but water from the tap.
 This image demonstrates the basics of how the magnesium salt water cell
operates. The concept is
This image demonstrates the basics of how the magnesium salt water cell
operates. The concept is
similar to that of the classic "fruit" battery. How ever the acid electrolyte fluid is replaced with sea water or regular salt water of just about any concentrations, The electrode, cathode
and anode dissimilar metals have been carefully chosen and studied, to produce the most current and voltage. Just a square inch of a magnesium anode is enough to produce over 1.5
volts with over 500ma for a few days or until mg anode completely dissolves from oxidation reaction. This can even light up an Incandescent light bulb. Comparable total power
output to that of a single AA 1.5 battery cell. This is some serious current for very few cents $$ to produce or "extract" energy No wonder this area (Sea Battery) Has had some interesting
attention lately.
Air-breathing fuel cells have a great potential as power sources for various electronic devices. They differ from conventional fuel cells in which the cells take up oxygen from ambient air
by active or passive methods. The air flow occurs through the channels due to concentration and temperature gradient between the cell and the ambient conditions. This technology is
usually used in hydrogen fuel cells. How ever. Nothing stops us from adapting this concept with the classic battery such as discussed previously with some slight modification to the
cathode electrode assembly.
A simple assembly can be constructed such as illustrated below to experiment with this concept.
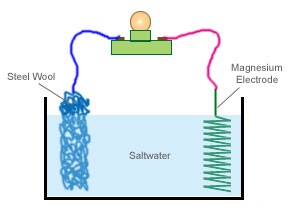 This setup is basic. But will work. You should get around 1 volt 100ma of
power out of
This setup is basic. But will work. You should get around 1 volt 100ma of
power out of
this experiment until the magnesium is completely dissolved. This setup is not efficient.
We use steel wool as the cathode and air breathing as the large rough surface area traps oxygen and "Breaths" well. Carbon sheet would work better! The cathode and anode
should be as close sandwiched together without actually shorting out. As in this example they are far apart. Greatly diminishing current output. How over some could argue
that 100ma with such a crude basic setup is remarkable.
Now how can we make this setup work better? This is done with the help of additional super thin layer of polymer electrolyte membrane between the two electrodes.
The closer together the more current output.
Here is a more practical complete version of this concept.
 As
you can see we are still using sea (salt) water. The electrodes are much
As
you can see we are still using sea (salt) water. The electrodes are much
closer together and separated with the thin solid polymer electrolyte membrane. This makes the cell much more practical in size and comparable to the equivalent output of
regular traditional bought battery.
With this kind of setup. A single cell of the size of around 1.5 inch, is enough to produce over 1 volt at over 1 amp. It some cases this setup can be good for a power output
of a couple watts based on my experiments.
For the relatively small size and almost free components and parts to operate. This is green energy and does not produce any harmful toxic byproducts as the end result. These
cells can also be stacked like regular power cells for more voltage and power requirements as needed.
Here is my version of this reproduction. Simple enough. Used mostly "lego" parts I had laying around the house. I ordered some of the raw parts online and cut my own
plates of magnesium with the help of hand held steel cutters. With various dimensions to fit the cells and experiment with different sizes and thickness.
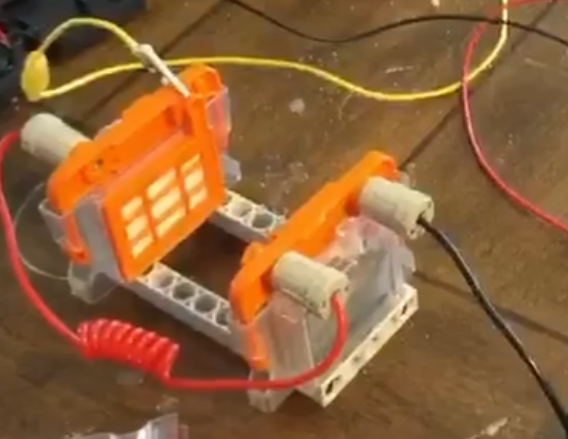 I insert regular salt water with the
help of the top hole and use the clear plastic storage "tank".
I insert regular salt water with the
help of the top hole and use the clear plastic storage "tank".
The snap in module holds the cathode on one side and the anode on the other and the polymer electrolyte membrane fabric next to a plastic grid so the air can pass and hold the water
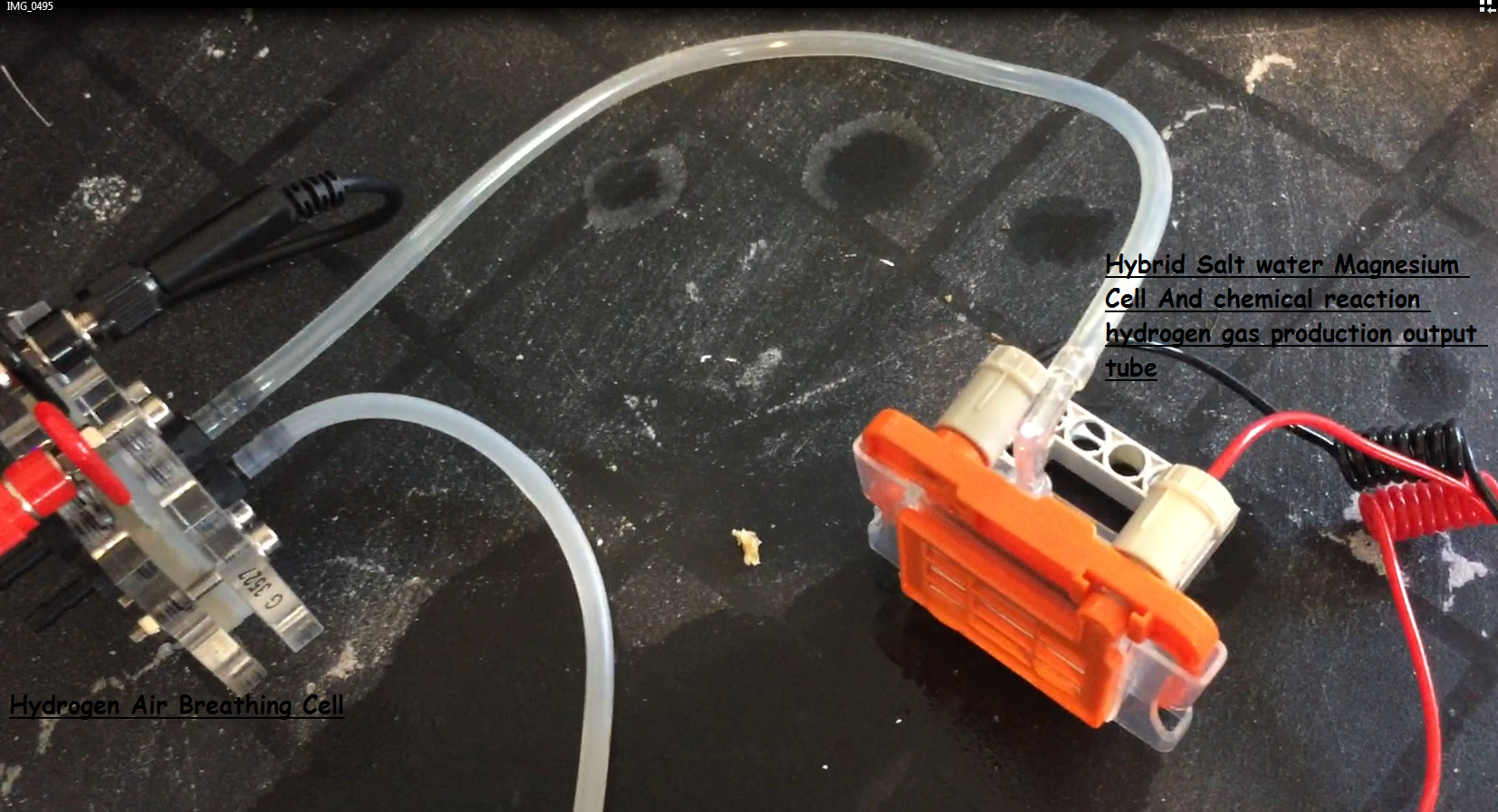
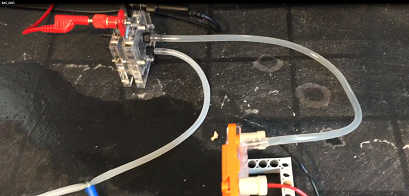
from instantly leaking from the other side making a big mess. I use two cells and I feed this voltage into a hydrogen generator.
With the help of the electrolyser. Hydrogen is produced. I feed this gas
with the help of clear plastic pipes
into a hydrogen air breathing cell. I am able to get some very good voltages with this method.
 Voltage
reading of around 7 volts at the hydrogen fuel cell stack.
Voltage
reading of around 7 volts at the hydrogen fuel cell stack.
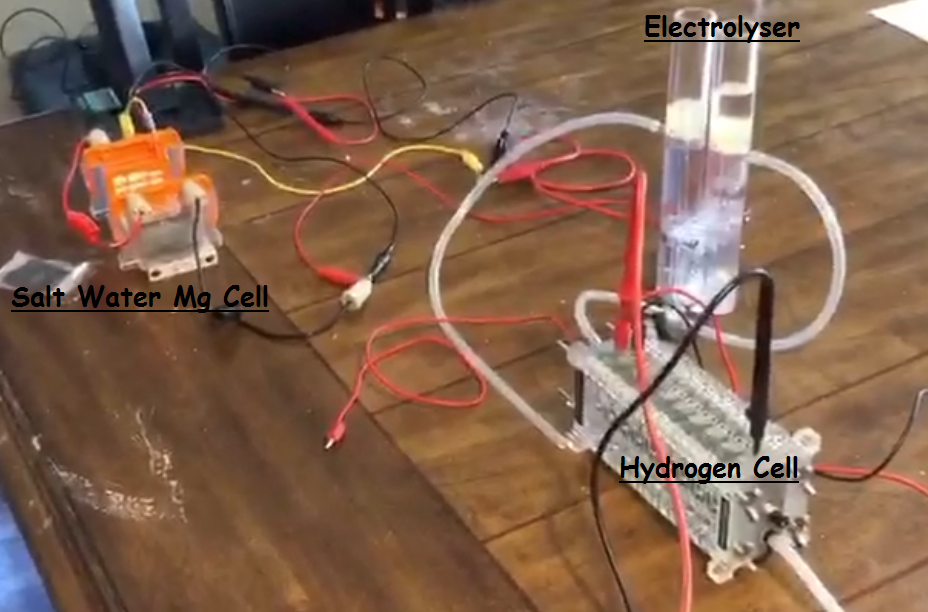
This also has no problems running fans (DC motors)

I had a great time learning and experimenting with these concept and ideas. Moving on with more research I learned more about chemicals and concepts that may be applicable
that I could use to modify and refine my process.
Acetic acid is mildly corrosive to metals including iron, magnesium, and zinc, forming hydrogen gas and salts called acetates. The elements potassium, sodium, lithium and calcium
are very reactive and they react with cold water to produce hydroxides
and hydrogen gas.
The elements magnesium, aluminium and iron are also considered as active
metals and they react with steam to produce oxides and hydrogen gas.
I used what I had laying around the house to experiment with. Acetic acid is basically just vinegar. I dripped the vinegar on various metals and watched for a reaction and noted
what ones gave a reaction or lack of and measured intensity of the reaction by observation. I found that with what I had to experiment with that the magnesium had to most
profound reaction. The stuff will fizz and heat up instantly. I'm not a chemist and I am sure other metals work well if not maybe even better. More experiment is needed with
finding good metals that react to vinegar and produce hydrogen. This is a very interesting "powerless" method of generating the hydrogen and decided to experiment with the
concept.
I filled a lab tube with a bunch of magnesium flakes I cut out by hand with the help of hand held steel sheet cutters. I then filled 1/4 of the tube with vinegar. Sealed the top
of the tube, The extra space inside to act as a hydrogen storage tank and when pressure builds up. Hydrogen gas leaves the top tube and enters the hydrogen fuel cell to convert
to DC voltage.
 Magnesium
flakes on the bottom causing a sever reaction with the vinegar.
Magnesium
flakes on the bottom causing a sever reaction with the vinegar.
Hydrogen quickly builds up in the sealed upper storage tank and pressure drives the hydrogen out the tube and into the fuel cell.

Plenty of voltage is being produced at the hydrogen fuel cell. Enough to drive motors and lamps.

Fuel Cell stack image below.
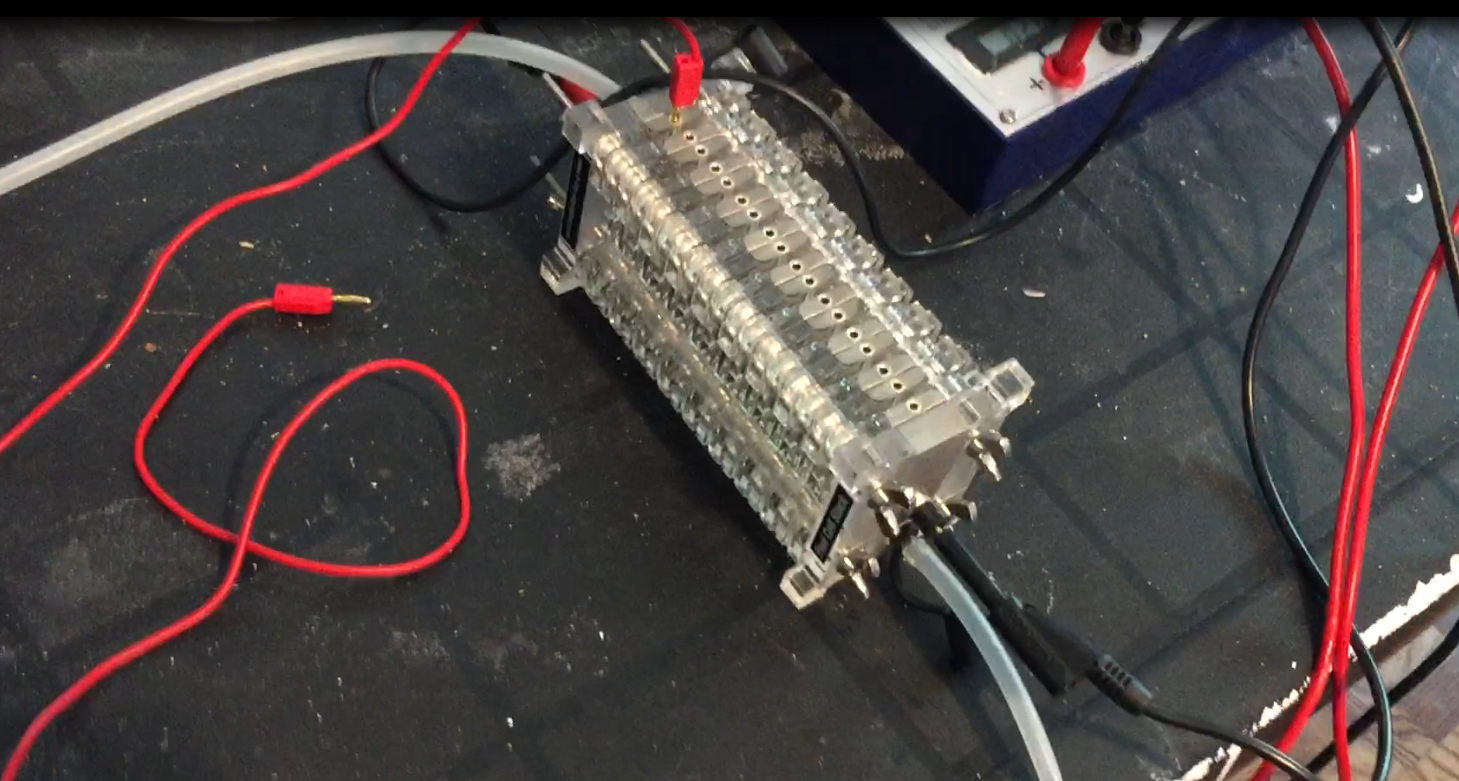
All this is being done with no "input" voltage. No electrolyser stage needed with the chemical reaction method! I pondered that I could
adapt this method to my previous salt water cell and eliminate the electrolyser stage all together by slight modification of my salt water
magnesium cell and turn it into a hybrid cell.
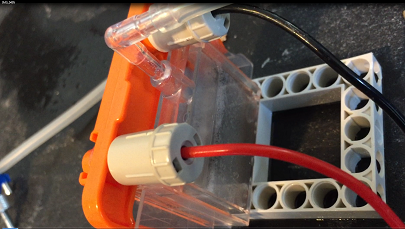 Now we have a few modifications. I
added a vinegar and salt water to the
Now we have a few modifications. I
added a vinegar and salt water to the
fluid. The logic is the salt water will react to the magnesium as normal and cause the previous reaction. How ever with the addition of
vinegar. This also creates very quick hydrogen as a byproduct, As result I have added a tube to the top where this bubbling hydrogen can
escape and feed a hydrogen cell as well. simultaneously. What is interesting in this configuration, two cells of completely different configurations
are operating at the same time working off each other but produce a totally independent voltage output. For example using very little or up the the
max current output each cell can put out does not linger the works of the other cell. Hydrogen keeps being produced at the same rate even if that
cell is utilizing strong current output or no current at all!
With the previous setup when using the electrolyser for hydrogen production. This uses considerable power amperage from the salt water cell.
All wasted in hydrogen production to feed the hydrogen cell. That power that could be used is mostly then lost.
The more power my requirement, The faster/more need for hydrogen gas production. If production can't keep up, the hydrogen cell can't output
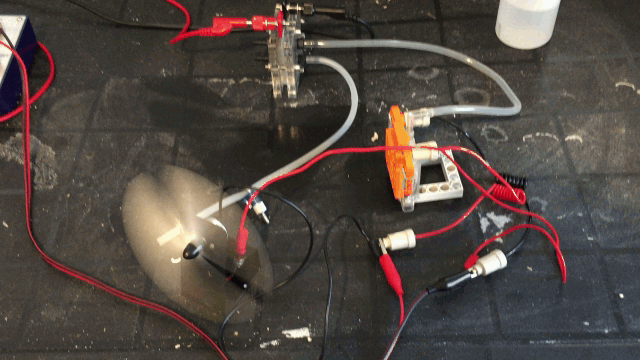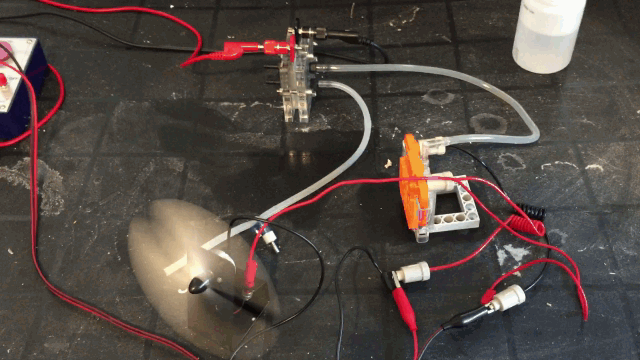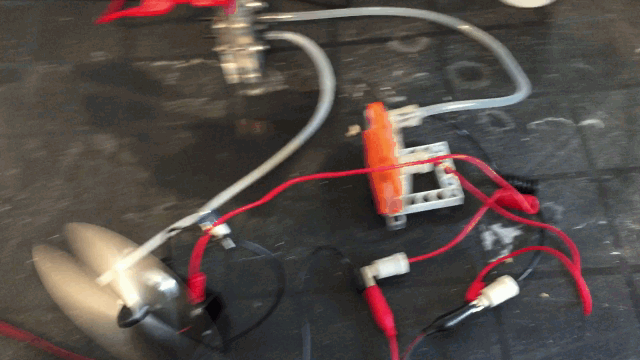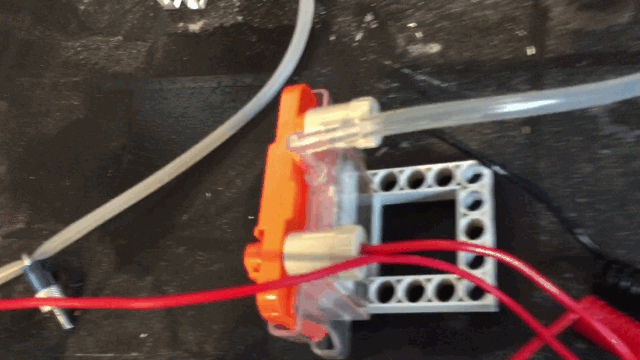
more power on time, Even as a hydrogen fuel stack setup. This hybrid cell addresses those issues. You can run both cells at once for more power!
without having to worry of over loading the input cell, rendering hydrogen (electrolyzer stage) useless, resulting in sudden total loss of power
from the system. This hybrid cell allows you to run both cells at once with 100% of available power to be available to be used. This is the most
inexpensive method of producing a couple watts at home of green energy for fractions of cents $$. With a little home chemistry and common
elements you can find around the house.
The image bellow demonstrates the hybrid cell producing voltage of its own and simultaneously producing hydrogen gas feeding a hydrogen cell.